What we do and how we do it
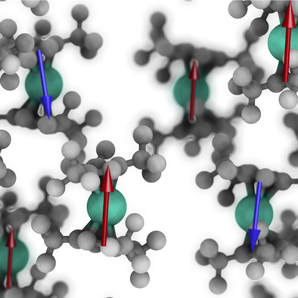
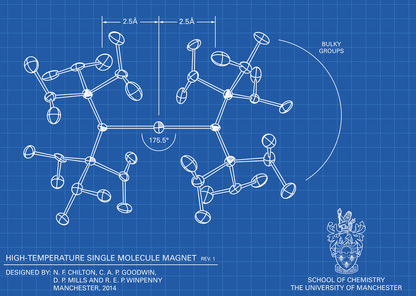
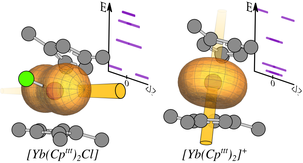
We are a synthetic chemistry group that focuses on f-element compounds - the lanthanides and actinides. We are interested in the synthesis of complexes with unusual geometrical features, which can engender interesting physical properties. These complexes are prepared by advanced Schlenk and glovebox techniques and are characterised by a plethora of techniques including single crystal X-ray crystallography, EPR, NMR, UV/Vis/NIR, FTIR, and Raman spectroscopies, magnetometric methods (eg. SQUID, Evan’s method), cyclic voltammetry, mass spectrometry and elemental analysis. Analytical data is supplemented by comprehensive theoretical studies on computationally demanding calculations of paramagnetic f-element systems with collaborators. All of these techniques combine to deepen our understanding of fundamentals of structure and bonding. We also explore the interesting further reactivity of these complexes.
Why f-element chemistry?
The f-elements have remarkable physical attributes that have been utilised in numerous consumer devices. Lanthanides find most industrial application in catalytic converters and petroleum refining, whilst their unique optical and magnetic properties have been exploited in high temperature superconductors, rare-earth magnets, magnetic resonance and fluorescence medical imaging, electric motors, lasers and phosphors. In addition, reactive lanthanide compounds have been frequently employed in fine chemical synthesis, e.g. hydroelementation, polymerisation and single-electron transfer (SET) reagents such as ceric ammonium nitrate (CAN) and samarium(II) diiodide. The inherent radioactivity of actinides has currently limited their usage to nuclear fuel cycles, but their molecular complexes often exhibit enhanced reactivity profiles that could be utilized in catalysis. However, despite these well-established applications, our knowledge of the f-elements lags behind the rest of the Periodic Table, thus we must first develop their fundamental chemistry to provide the necessary insights for future technologies.
We are a synthetic chemistry group that focuses on f-element compounds - the lanthanides and actinides. We are interested in the synthesis of complexes with unusual geometrical features, which can engender interesting physical properties. These complexes are prepared by advanced Schlenk and glovebox techniques and are characterised by a plethora of techniques including single crystal X-ray crystallography, EPR, NMR, UV/Vis/NIR, FTIR, and Raman spectroscopies, magnetometric methods (eg. SQUID, Evan’s method), cyclic voltammetry, mass spectrometry and elemental analysis. Analytical data is supplemented by comprehensive theoretical studies on computationally demanding calculations of paramagnetic f-element systems with collaborators. All of these techniques combine to deepen our understanding of fundamentals of structure and bonding. We also explore the interesting further reactivity of these complexes.
Why f-element chemistry?
The f-elements have remarkable physical attributes that have been utilised in numerous consumer devices. Lanthanides find most industrial application in catalytic converters and petroleum refining, whilst their unique optical and magnetic properties have been exploited in high temperature superconductors, rare-earth magnets, magnetic resonance and fluorescence medical imaging, electric motors, lasers and phosphors. In addition, reactive lanthanide compounds have been frequently employed in fine chemical synthesis, e.g. hydroelementation, polymerisation and single-electron transfer (SET) reagents such as ceric ammonium nitrate (CAN) and samarium(II) diiodide. The inherent radioactivity of actinides has currently limited their usage to nuclear fuel cycles, but their molecular complexes often exhibit enhanced reactivity profiles that could be utilized in catalysis. However, despite these well-established applications, our knowledge of the f-elements lags behind the rest of the Periodic Table, thus we must first develop their fundamental chemistry to provide the necessary insights for future technologies.
Collaborators:
UoM
We work with many groups at Manchester, including:
Prof. Nik Kaltsoyannis
Prof. Steve T. Liddle
Dr Louise S. Natrajan
Prof. David J. Procter
Dr Jonathan Skelton
Magnets group:
Dr Nicholas F. Chilton
Prof. David Collison
Prof. Eric J. L. McInnes
Dr Floriana Tuna
Prof. Richard E. P. Winpenny
Other institutions
We work with a wide range of external collaborators. We have recently published work with the groups below:
Dr Stephen Sproules (Glasgow)
Prof. Kyle Lancaster (Cornell)
Dr Rodolphe Clerac (CRPP-CNRS)
Dr Stefano Caretta (Parma)
Dr Tatiana Guidi (ISIS)
Prof. William J. Evans (UCI)
Dr Andrew Kerridge (Lancaster)
Prof. Laurent Maron (Toulouse)
UoM
We work with many groups at Manchester, including:
Prof. Nik Kaltsoyannis
Prof. Steve T. Liddle
Dr Louise S. Natrajan
Prof. David J. Procter
Dr Jonathan Skelton
Magnets group:
Dr Nicholas F. Chilton
Prof. David Collison
Prof. Eric J. L. McInnes
Dr Floriana Tuna
Prof. Richard E. P. Winpenny
Other institutions
We work with a wide range of external collaborators. We have recently published work with the groups below:
Dr Stephen Sproules (Glasgow)
Prof. Kyle Lancaster (Cornell)
Dr Rodolphe Clerac (CRPP-CNRS)
Dr Stefano Caretta (Parma)
Dr Tatiana Guidi (ISIS)
Prof. William J. Evans (UCI)
Dr Andrew Kerridge (Lancaster)
Prof. Laurent Maron (Toulouse)
We thank The University of Manchester and the Engineering and Physical Sciences Research Council (EPSRC) for funding.